what is a disadvantage of submerged biofilters relative to most other biofilters?

Comparison of Unlike Biofilter Media During Biological Bed Maturation Using Common Carp as a Biogen Donor
Department of Ichthyology and Aquaculture, Academy of Warmia and Mazury, Al. Warszawska 117A, PL 10-701 Olsztyn, Poland
*
Author to whom correspondence should be addressed.
Received: 3 December 2019 / Revised: five January 2020 / Accepted: seven Jan 2020 / Published: 15 January 2020
Abstract
This experiment analysed the functioning of submerged and dripping biological filters with three types of filling: commercial plumbing equipment HXF12KLL (CF), 2 innovative polypropylene aggregates (PPA) and polyethylene spiral caps for PET bottles (PSC). The experiment determined the time needed to reach full filter functionality at the maturation stage, the fourth dimension needed to start successive stages of the nitrification process and the maximum concentration of each nitrogen compound in water in the recirculation systems. The filter operation characteristics subsequently the maturation stage were also examined. These issues are crucial during the preparation and launch of new aquaculture facilities. A literature analysis indicated that the ability of biological filters to oxidise nitrogen compounds is affected by a number of factors. Studies conducted at various centres accept covered selected aspects and factors affecting the effectiveness of biological filters. During this study, the model fish common bother (Cyprinus carpio) was used. The current experiment involved test of biological filter maturation and operation during the carp fry rearing stage, which allowed the biofilter performance characteristics to be determined. At the third day of the experiment, the ammonium concentration reached approximately iii mg NH4-N/dmiii. It remained at this level for 10 days and afterward decreased beneath 0.25 mg NH4-North/dm3. The maximum nitrite concentration ranged from eleven.seven mg/dmiii to 20.ix mg NO2-N/dm3 within ix to 20 days and later decreased with time. Nitrate concentrations were seen to increase during the experiment. The all applied biofilter media showed possibility to be used in commercial aquaculture systems.
1. Introduction
Annual fish and seafood consumption has been increasing steadily. The amount of fish and seafood obtained globally is express and annual output has remained at 90 million tonnes for the past decade. For this reason, any increase in the amount of fish and seafood is associated with aquaculture and its dynamic growth. The production of aquaculture accounted for 44% of the total output in 2014 [one,2]. Installations used for creature product can be classified in regard to the caste of water recirculation: (1) open/flow-through systems, in which water is used once; (2) semi-open/semi-closed systems, in which h2o is used multiple times earlier being removed from the organization, and (three) closed systems, in which just water loss is replenished. Higher degrees of water recirculation require more complex systems to purify it. RAS denotes technologies of repeated water reuse in a closed organisation. Nevertheless, water loss in convenance systems needs to be replenished for multiple reasons, not but because of evaporation. To minimise the need for replenishing h2o loss, RAS systems are equipped with complex water treatment and purification systems, including mechanical and biological filters, UV sterilisation devices, water ozonation systems and others [iii,4]. The virtually important of these systems are biological filters, which control toxic nitrogen species and are necessary for RAS performance [iii,iv,v]. The fast increasing of aquaculture product in RAS is not possible without new developed technologies. One of the primary focuses is media for biofilters, upon which the effectiveness of nitrification is dependant. This likewise an influence of biofilter costs, then new and cheaper biofilter media are necessary to involve in farther aquaculture production growth [1,two,3,4,five].
The removal of toxic nitrogen from h2o is affected by nitrification, consisting of the biological oxidation of ammonium nitrogen to nitrite (III) nitrogen, followed by oxidation of the latter to nitrate (V) nitrogen. A crucial role in the nitrification process is played past Nitroso- (oxidation of ammonium to nitrite) and Nitro- (oxidation of nitrite to nitrate) nitrification bacteria [6,7]. Total ammonium nitrogen (TAN) is 1 of the major limiting factors in the design and operation of RAS systems [8] and is i of the metabolites which is formed in the digestion of proteins and the transformation of amino acids [9] given to fish with feed. Information technology occurs as a sum of two species: dissociated (ammonium ion, NH4 +) and undissociated (ammonia, NH3) [8,10]. Ammonium nitrogen in its dissociated form is relatively non-toxic, whereas the undissociated form is highly toxic [8,9,11,12,13,xiv,15,16,17]. For this reason, information technology must be controlled [17] and removed from the system or oxidised to a less toxic nitrogen course [8,xviii]. Ammonia levels in RAS systems are controlled by nitrification [17]. The NH4 +/NHthree ratio depends mainly on the environment's pH. The corporeality of toxic NH3 increases forth with college pH, depending on the temperature, pressure and salinity. Excessive amounts of ammonia atomic number 82 to tremors in fish, coma and death [x]. The ammonia removal process is afflicted past a number of factors. The about of import of them is the availability of oxygen (4.57 yard of oxygen is needed to oxidise 1 g of ammonium nitrogen) and the charge per unit of its diffusion into the biomembrane [8]. A decrease in water saturation with oxygen results in inhibition of ammonium nitrogen oxidation [19,xx]. Important factors also include temperature, pH, salinity and organic matter brunt on the biological filter [viii]. Nitrite nitrogen are intermediate compounds in nitrification [six,7]. Due to their toxic effect on fish, they are an every bit important factor in fish breeding as ammonium nitrogen [7,14,21,22] and are responsible for chronic diseases leading to fish death [7]. Nitrate nitrogen is the last step in the nitrification process [6,7] and has long been regarded as harmless. Recent studies have shown that it should be taken into account when optimising fish breeding. This nitrogen form is relatively harmless, simply long-term exposure is suspected of having deadly toxic effects [21,23], although farther studies are needed [23]. Analysis of the nitrification procedure gives the information if the biofilter is matured. In RAS equipped with such a biofilter, the level of ammonium and nitrite concentrations are low and stable [eighteen]. It is especially important for finfish species, which are sensitive to toxic nitrogen compounds, like salmonids [4,half-dozen,7,11,12].
The kinetics of the reaction were not studied during the experiment, because the purpose of the study was to examine whether the practical experimental fillers for biological filters are useful in aquaculture. The reaction kinetics report is the side by side step after confirming the suitability of the filling used. The operation of modern aquaculture systems and their further dynamic growth requires the continuous development of new technologies [24]. Information technology is as important to go on in mind while developing new technologies or solutions that biological filter operation is affected past a number of factors, e.g., dissolved oxygen and the rate of its improvidence into the biomembrane, temperature, pH, salinity and organic matter burden on the biological filter, which is not ever reflected in laboratory tests [7,25,26,27]. Considering the above, a two-stride experiment was conducted on a semi-commercial calibration, in which submerged and dripping biological filters with iii different fillings were used, which are different from each other. CFs (commercial fitting HXF12KLL) have been designed to obtain the largest possible area in a unit of volume. In add-on, their spatial structure allows the costless menses of water through the centre of individual fittings, which effectively supports nitrification (nitrifiers have constant admission to nitrogen compounds). PSCs (polyethylene screw caps for PET bottles) have more than three times smaller expanse per unit volume, merely due to their shape, the inflow of nitrogen compounds to the biofilm is profoundly facilitated. PPAs (polypropylene aggregate) have the to the lowest degree regular shape. A microscopic photograph reveals a lack of internal spaces on which a bacterial biofilm could develop. Nevertheless, the outer construction has numerous recesses and protrusions, which are very diverse and provide favourable conditions for the development of nitrifiers. The main donor of nitrogen compounds were mutual carp Cyprinus carpio juveniles. The study examined the dynamics of nitrogen compound transformations during biological bed maturation and matured bed operation. Moreover, increasing the daily nutrient dose allowed the bed operation to be examined with increasing loads.
A organisation with submerged filters with commercial fillings was used as a control. During the experiment, the hypothesis was verified that the use of polypropylene aggregate and polyethylene screw caps for PET bottles as fillers for biological filters will attain the same furnishings every bit the use of commercial fittings HXF12KLL.
2. Materials and Methods
The experiment lasted threescore days in the submerged biological bed variant (ii days of fish acclimatisation and 58 days of the water parameter measurements) using tap water. The experiment lasted 45 days in the dripping biological bed variant (two days of fish acclimatisation and 43 days of the h2o parameter measurements). The biological bed maturated during this fourth dimension, which allowed the dynamics of nitrogen chemical compound transformations in a maturated bed system to be examined. Moreover, increasing the daily nutrient dose allowed the bed operation to be examined with an increasing load. Ammonium, nitrites and nitrates' concentration in tap water at the beginning of the experiment was 0.045 mg Due north-NH4/dm3, 0.027 mg N-NO2/dm3 and 0.872 mg N-NOthree/dm3.
2.1. Experimental Weather condition
2.one.1. RAS Systems
The experiment was conducted in 4 identical semi-open RAS systems with 625 dm3 volumes each (daily refill—150 dm3) modelled on devices used past Sikora et al. [18]. Three types of biological filter fillings were examined:
- -
-
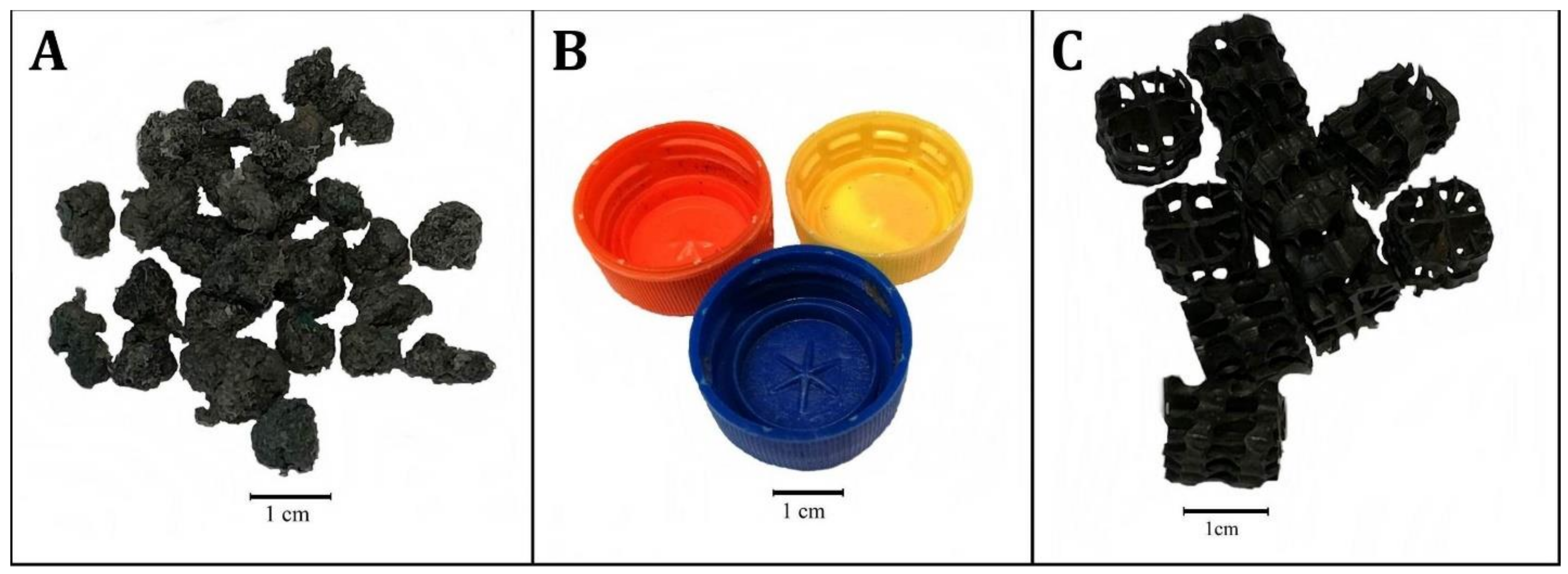
Polypropylene aggregate (PPA) (active area 300–500 k2/yardthree —manufacturer's data) (Figure 1A).
- -
-
Polyethylene screw caps for PET bottles (PSC) (active surface surface area ca. 300 mtwo/m3) (Effigy 1B).
- -
-
Commercial fittings HXF12KLL (CF) (active area 859 m2/thousand3, Stöhr GmhH & Co. KG, Marktrodach, Germany) (Effigy 1C).
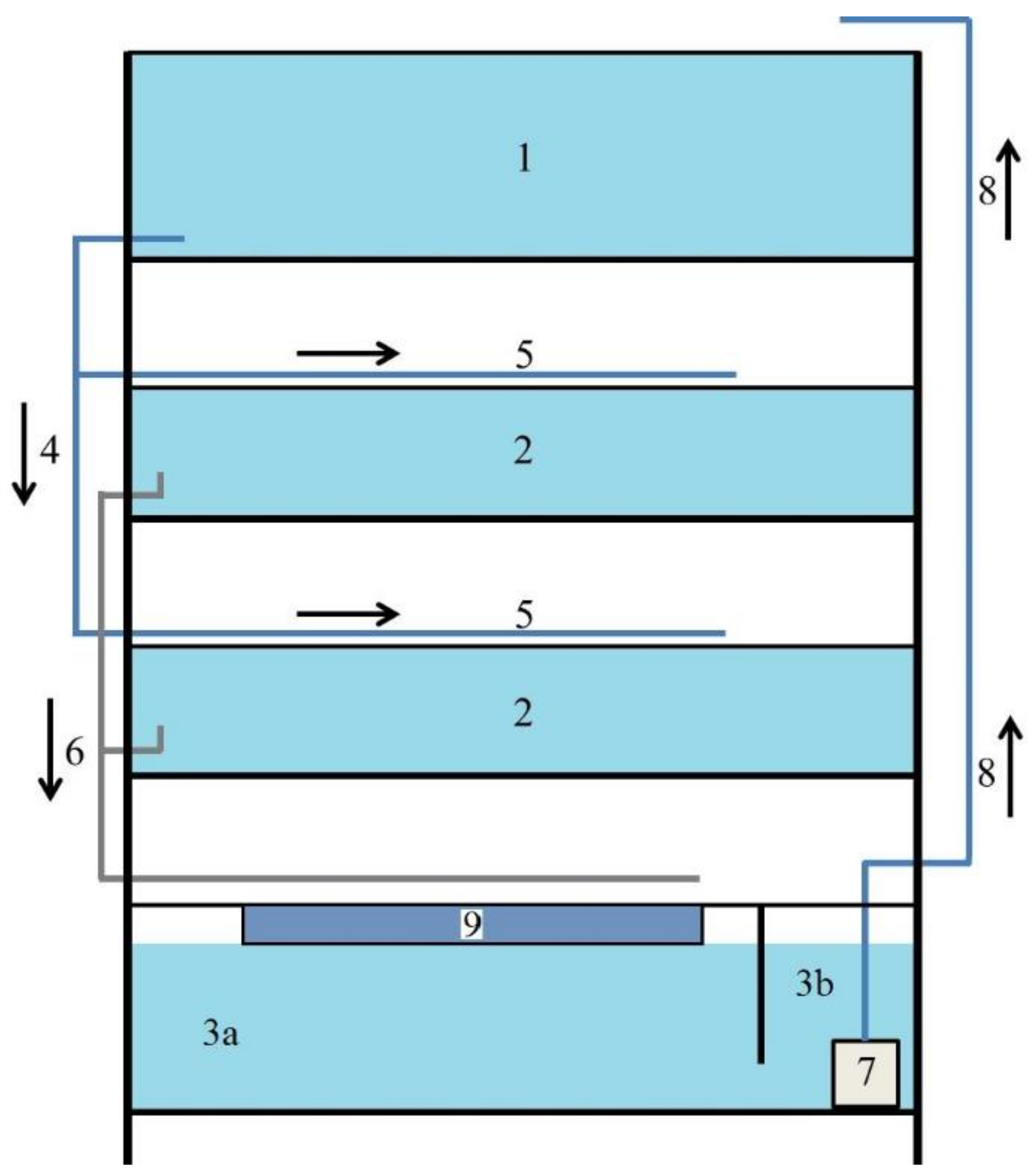
Each RAS system consisted of: an upper retention reservoir, a 2-chamber lower retention reservoir (sedimentation chamber, pump bedchamber) and 2 rearing tanks. The systems were also fitted out with heaters, thermostats and aeration systems. The biological filter filling volume was 30 dm3. The specifics surface area of the biological filters used was: PPA—ca. 12 1000ii, PSC—ca. 9 m2, CF—ca. 25.5 mtwo. For submerged filters (Figure 2), the filling was placed direct in the sedimentation sleeping accommodation. The filling was suspended above the sedimentation chamber in the variant with dripping filter (Figure 3), which had sprinklers mounted above them.
The systems were started five days before the fish were put in them to stabilise the weather in the recirculation systems. The circulating water temperature was 25.0 ± 0.ane °C. Before the experiment, the systems were first thoroughly stale, disinfected with potassium permanganate at vii.five g per run and then rinsed with water from the water supply organisation for 24 h.
2.1.2. Fish
Common carp juvenile (Cyprinus carpio), bred at the Department of Lake and River Fisheries of the University of Warmia and Mazury in Olsztyn and cultured under controlled weather condition, were used in all experiment as a source of nitrogen compounds. Out-of-season carp breeding was carried out in accordance with the methodology described by Kucharczyk et al. [28], with the insemination method modified by Kucharczyk et al. [29] for burbot (Lota lota), ide (Leuciscus idus) and asp (Aspius aspius). The carp larvae were reared in a recirculation organisation at 25 ± 0.one °C and fed brine shrimp (Artemia salina) nauplii for the outset 25 days. Bogus feed with a granulation of 0.5–1.1 (Skretting, Norway: raw poly peptide content 54%, fat content 18%) was introduced after that time.
In the submerged biological bed variant, 1379 ± 49 yard (average ± SD) of fry with a weight and unit length of 3.76 ± ane.55 g (boilerplate ± SD) and 59.00 ± 8.77 mm (average ± SD) were placed in the RAS systems. In the dripping biological bed variant, yet, 1400.00 ± 0.00 1000 fry with a unit weight and length of 16.xxx ± 4.80 chiliad and 96.twoscore ± 9.65 mm were placed in the RAS systems. All fish used during the experiment are juvenile forms of mutual carp.
The fish (carp) were fed twice daily (at eight.30 and fifteen.00) during the experiment with an artificial feed with a granulation of one.1 (Skretting, Norway: raw protein content 54%, fatty content 18%) and 1.nine mm (Skretting, Kingdom of norway: raw protein content 50%, fat content twenty%), with a single feeding lasting thirty min. The feeding dose was set at 3% of the daily biomass (half of the daily feeding dose was provided in one feeding). Subsequently, the dose was increased daily by 3% of the initial fish biomass [xxx,31]. The initial daily feed dose was 20.9 ± 0.5 g (average ± SD).
Sodium chloride was added to circulating water offset on the mean solar day the nitrite nitrogen concentration reached 1 mg N-NO2/dmiii. Nitrite shows of affinity to the Cl-/HCOiii - ion exchange. Every bit a consequence, a function of the nitrite is taken up by fish instead of chloride. Increment of chloride concentration in the water reduces nitrite uptake by fish [32].
2.2. Water Quality Measurements
Water tests started two days afterwards the fish were placed in the systems. The water in each system was examined at 11:00 (samples were taken from the sprinkler). Hach Lange cuvette tests with a dedicated DR 5000 spectrophotometer were used for the water testing [18]:
-
Ammonium—cuvette exam LCK 304
-
Nitrite—cuvette test LCK 341
-
Nitrate—cuvette examination LCK 340
Daily ammonium assays were conducted for the initial 17 days of the experiment. The aim of this test was to check whether the compound accumulates in the water in excessive amounts. After that time, measurements were conducted every three days to monitor the full ammonium concentration in the circulating water. The nitrite concentration was checked daily throughout the experiment to make up one's mind the time needed to start stage I of nitrification (oxidation of ammonia to nitrites). The nitrate concentration in the water was tested daily for the showtime 28 days, after which measurements were conducted every two days to make up one's mind the fourth dimension needed to outset stage Two of nitrification (oxidation of nitrite nitrogen to nitrate nitrogen).
Moreover, the following water parameters in the bed were measured at 11.00:
- -
-
pH—using a portable pH-meter manufactured by Oxyguard,
- -
-
Oxygen concentration (mg Oii/dm3, saturation)—using an oxygen probe, a portable Polaris 2 manufactured past Oxyguard,
- -
-
Temperature—using an oxygen probe, a portable Polaris ii manufactured by Oxyguard,
Moreover, the post-obit h2o parameters were tested before the forenoon and afternoon feeding:
- -
-
Temperature—a probe combined with a thermostat controlling heater work,
- -
-
pH—using a portable pH-meter manufactured by Oxyguard,
- -
-
Oxygen concentration (mg Oii/dm3, saturation)—using an oxygen probe, continued with the Full Commander system manufactured past Oxyguard.
2.3. Fish Measurements
The fish were measured (average weight and average total length) at the beginning and end of the experiment. Thirty fish were collected randomly and weighed with an belittling balance (KERN & Sohn GmbH, Balingen, Germany) with an accurateness of 0.1 mg. The fish body lengths were measured with a calliper MEGA 20513 (Profix, Warsaw, Poland) with an accurateness of 0.01 mm. Due to the fish size, their bodies were measured after the experiment with a ruler with an accuracy of 1 mm. Moreover, the fish biomass was determined at both the beginning and end of the experiment (analytical rest KERN & Sohn GmbH, Germany). The fish were anaesthetised during the measurements with an MS222 anaesthetic at a concentration of l ppm.
2.four. Statistical Assay
The dynamics of nitrogen compound (ammonia, nitrites, nitrates) transformations were examined with a Kruskal–Wallis ANOVA test on ranks (p > 0.05) and subsequently by multiple comparisons of mean ranks (p > 0.05) for all samples. The distribution normality was verified with the Shapiro–Wilk examination (p > 0.05) before the ANOVA test was performed. All results were analysed statistically using Statistica xiii.1 software (StatSoft, Tulsa, Oklahoma, U.s.a.). In addition, in lodge to analyse the nature of individual stages of nitrification, regression equations were performed and the correlation coefficient (R) was calculated.
iii. Results
three.1. Variant I—Use of Submerged Biological Filter
3.i.1. Ammonium Nitrogen
At the beginning of the experiment, the ammonium concentration increased rapidly in all experimental RAS and was about 3 mg N-NH4/dmthree from day three (Figure 4).
High ammonia levels lasted for several days and and so apace decreased. After this period, until the end of the experiment, no major increases in ammonium were recorded in RAS circuits (Table 1).
3.1.two. Nitrite Nitrogen
In all RAS, nitrite levels increased for several dozen days until the maximum concentration was reached. After this time, the concentrations decreased until they reached a relatively low and stable level, which remained until the stop of the experiment (Figure 5; Table 2).
3.1.3. Nitrate Nitrogen
The nitrate concentration in the arrangement with PPA every bit the biological filter filling increased until day 53 and reached 32.05 mg Due north-NOiii/dm3. It began to decrease afterwards. A similar trend was observed in the other two experiment variants. The concentration in the PSC system too rose until twenty-four hour period 53 to reach a like level (34.35 mg N-NO3/dm3). The highest concentration in the system with CF filling was higher than in the other two systems (50.40 mg N-NOiii/dm3) and information technology was measured during a shorter time (day 44) (Effigy 6).
3.1.4. Other H2o Parameters
The average water temperature in the system with PPA was 24.8 ± 0.four °C, average pH—8.01 ± 0.15, whereas the average concentration of oxygen dissolved in water and saturation were 6.half dozen ± 0.7 mg O2/dm3 and 79.8 ± viii.7%, respectively. The aforementioned parameters in the PSC system were: water temperature 24.8 ± 0.3 °C, pH 8.03 ± 0.15, dissolved oxygen concentration half dozen.7 ± 0.vii mg O2/dm3, saturation 82.4 ± 8.6%. In the system with CF filling, the parameters were: water temperature 24.8 ± 0.6 °C, pH 8.06 ± 0.14, dissolved oxygen concentration 6.eight ± 0.6 mg O2/dm3 and saturation 81.6 ± vii.2%.
3.ii. Variant 2—Apply of Dripping Filters
The organisation with a submerged filter with CF filling (CFsf) was used again as the command in the second part of the experiment with dripping filters. There was a failure and leak in the arrangement with the dripping filter filled with CF during this office of the experiment. The organisation was restarted in accordance with the procedure presented in the methodology.
3.2.i. Ammonium Nitrogen
Every bit in the case of submerged filters, the concentration of ammonium in the experimental RAS systems increased rapidly and from solar day 3 it was about 3 mg Due north-NH4/dmthree (Figure seven).
High ammonia levels lasted for several days and so apace decreased. Subsequently this period, no pregnant increases in RAS ammonium were noted until the end of the experiment (Tabular array 3).
Only in the system in which a failure occurred were other characteristics of ammonium concentrations noted. The highest concentration in the system with CF was recorded on day 3—1.230 mg N-NHfour/dm3—and it decreased afterwards. The ammonium concentration decreased below 0.three mg N-NH4/dm3 after day 8 and until the finish of the experiment information technology fluctuated, with an average concentration of 0.188 ± 0.047 mg N-NH4/dm3 (Figure 7).
3.ii.ii. Nitrite Nitrogen
In all RAS, nitrite levels increased for several dozen days, until the maximum concentration was reached. Afterwards this time, the concentrations decreased until they reached a relatively low and stable level, which remained until the finish of the experiment (Figure viii; Tabular array four).
three.two.three. Nitrate Nitrogen
The nitrate concentration in the system with PPA as the biological filter filling increased until solar day 44 and reached 32.150 mg North-NOthree/dmiii. It decreased slightly afterwards. A similar tendency was observed in the arrangement with PSC. The highest concentration of 30.00 mg N-NOthree/dm3 was observed on day forty and it decreased slightly subsequently. A unlike state of affairs was observed in the system with CF. The concentration of nitrate nitrogen increased to 29.800 mg N-NOthree/dm3 on day 36; afterwards it decreased until the terminate of the experiment, reaching 25.00 mg N-NO3/dm3 on the final day. In the organization with CFsf, the nitrate concentration increased until twenty-four hours 32 and reached 32.15 mg N-NO3/dm3 and it decreased slightly later on (Figure 9).
3.2.iv. Other Water Parameters
The average water temperature in the system with PPA was 24.9 ± 0.1 °C, average pH—8.84 ± 0.10, whereas the boilerplate concentration of oxygen dissolved in water and saturation was 7.three ± 0.iii mg O2/dmiii and 88.9 ± 3.8%, respectively. The aforementioned parameters in the organisation with PSC were: water temperature 24.nine ± 0.2 °C, pH viii.82 ± 0.10, dissolved oxygen concentration 7.5 ± 0.four mg O2/dm3, saturation 92.1 ± iii.9%. In the system with CF, the parameters were: water temperature 25.0 ± 0.3 °C, pH 8.83 ± 0.12, dissolved oxygen concentration 7.0 ± 0.4 mg Otwo/dm3 and saturation 85.7 ± 4.7%. The parameters in the control system (CFsf) were: water temperature 24.ix ± 0.1 °C, pH 8.74 ± 0.15, dissolved oxygen concentration 7.0 ± 0.iii mg Otwo/dm3 and saturation 86.1 ± 2.seven%.
3.three. Statistical Analysis of Results
The results do not take a normal distribution (p < 0.05, Shapiro–Wilk test). A statistical assay of the results using a Kruskal–Wallis ANOVA rank test revealed significant differences between the RAS systems (p < 0.05). Detailed results are provided in the table (Tabular array 5).
Analysis of the results of ammonium concentrations in individual RAS allowed the determination of 2 periods of a different graphic symbol for which regression equations take a different form. Later on placing the fish, the concentration of ammonium in individual systems increased. High levels of ammonium were noted from day 3, followed by depression ones. In that location was 1 day of intermediate values between periods. Increases and decreases of ammonium concentration occurred rapidly. In the example of a organization in which a failure occurred and it became necessary to restart it instead of a period with loftier concentrations, there was a period during which the concentration gradually decreased (Table 1 and Table iii).
The analysis of nitrite concentration resulting in individual RAS allowed the determination of four periods with a dissimilar nature of the form. These periods for different RAS systems take a different duration merely testify a like nature. In the first menstruum, the regression equation describing the increase in concentration takes the course of an exponential role. In the 2d menstruation, the concentration initially rose, reached the maximum level and began to reject and the equation takes a polynomial form. In the third menses, characterized past a decrease in concentration, the equation takes on a power form. In the 4th flow, nitrite concentrations remain at the aforementioned level without significant decreases or increases, and the equation again takes on a polynomial form (Tabular array 6a,b).
The concentration of nitrates in RAS systems show a similar tendency. Afterwards the initial slow increase in concentration, accelerated growth followed and was and so inhibited. An analysis of the obtained results showed a decrease in nitrate concentration at the cease of the experiment, which is reflected in the derived regression equations (Table 7).
3.four. Fish
During the experiment, no mortality was observed in the reared fish.
4. Discussion
In the experiment, the dynamics of changes in nitrogen compounds in the water used for rearing fish in semi-closed RAS were analysed in detail. The dynamics of nitrogen compounds (ammonium, nitrite, nitrate) during the maturation of biological filters also as during the operation of mature filters were examined. Furthermore, the usefulness of PPA and PSC as fillings for biological filters was demonstrated. Due to the differences in construction, all 3 fillings accept their own advantages and disadvantages. Therefore, when comparison fillings, the analysis was based on the analysis of changes in concentrations of nitrogen compounds in water and the time needed to achieve total filter functionality. Comparing structural parameters, especially like in terms of the size of the active surface of PSC and PPA fillings, could be subjective. The bear on of structural parameters should be examined at a later stage of the study, in which the impact of private factors (eastward.thousand., oxygen, temperature, suspense solids, diffusion charge per unit, etc.) would be analysed on the dynamics of the nitrification procedure. H2o commutation is necessary in aquaculture farms using a closed water cycle equipped with biological filters without denitrification due to e'er increasing level of nitrate. Denitrification is the process of reducing nitrates to gaseous nitrogen. It is an anaerobic process that can sometimes occur in biological filters overloaded by biogens. The corporeality of water needed varies depending on several factors. The most of import is the increment in nitrate concentration, which in loftier concentrations shows adverse effects on aquatic organisms [21,23,33]. At the start-up stage of RAS facilities, water change is required for a similar reason. High concentrations of toxic forms of nitrogen occurring during the maturation of biological filters require the use of water changes not only to supplement evaporation. In the experiment, a i-fourth dimension top-up of 150 dm3 daily was used. This immune for the dilution of nitrogen compounds and while supplementing calcium compounds in water, it also allowed better observation of the dynamics of nitrogen compound transformation processes. An of import factor was also the use of common carp—a model species which is considered to exist relatively resistant to adverse environmental rearing conditions [34].
Mechanical and biological filters must piece of work together for the proper functioning of aquatic organisms [seven,26,27,35,36]. The importance of the filtration process becomes significant when the breeding facility is built in RAS engineering science [22,37,38,39,40,41,42]. For the proper class of biological purification in the nitrification procedure, the cooperation of two groups of microorganisms is required. The commencement group oxidizes ammonia to nitrites and the other group oxidizes the formed nitrites to nitrates [6,7,43,44,45]. By analysing the concentration of particular forms of nitrogen in the h2o used during the commissioning of convenance facilities, it is possible to observe individual stages of nitrification and thus make up one's mind whether the biological filter has gained functionality [xviii,45]. The unpredictability of the maturation process was shown in an experiment carried out by Pulkkinen et al. [46] illustrating the effect of the type of biological filter used (stock-still and moving bed bioreactors) on nitrification in recirculating aquaculture systems. In that experiment, despite the use of filter fillings operating for six months, the faulty operation of the bed was observed, which was revealed by an initially high concentration of nitrites, whose low level was accomplished but after well-nigh eleven weeks. According to these authors, such a situation was acquired past disturbances in the bacterial limerick produced by the shock of transportation to a laboratory. This indicates the exceptional sensibility of nitrifying bacteria to variable environmental weather. Due to the high concentration of toxic nitrogen compounds, fish rearing in circuits with young biological filters threatens the loss of fish beingness reared. This is especially true for salmonids [47]. Therefore, species resistant to elevated concentrations of nitrogen compounds should be used at the start-up phase of aquaculture facilities.
The experiment using fish as a source of compounds needed in the nitrification process immune observation of the work of the biological filter in conditions simulating real fish breeding [18]. Research carried out in this style is very of import to properly carry out the maturation procedure of the biological filter and obtain a filter adapted to the given conditions in RAS systems. A review of the literature has shown that this approach to the topic is rare [18,48]. Inquiry using constructed solutions with nitrogen compounds dominates the field [twoscore,49,50]. In this experiment, carp was used as a model species [34] with loftier metabolism [31].
During the experiment, all systems noted a rapid increase in the ammonium content in water to approximately iii mg Due north-NHfour/dm3 (Figure 4 and Figure 7; Table 1 and Table 3). The increase in ammonium concentration resulted from placing the fish in the water cycle and starting feeding them. It should exist emphasized that carp is a species with very loftier metabolism [31]. This level persisted from 4 days (submerged filter, CF) to 9 days (submerged filter, PSC) (Figure 4). In other filters, the duration of elevated ammonium concentration in the circuits was seven–8 days. After this period, at that place was a rapid, several-fold subtract in ammonium concentration in the RAS. It was causeless that the process involving the drying of the circuits and their disinfection through the use of potassium permanganate will eliminate nitrifying bacteria. The abrupt increase in ammonium has proved this assumption. Low concentrations persisted until the end of the experiment. In the arrangement that was restarted, the distribution of ammonium concentrations was different (Figure seven). The first recorded results were almost 60% lower than in other systems, and there was a rapid decrease in ammonium concentration. The fall curve was much milder. There is a discrepancy between the results obtained for two systems in which submerged filters filled with CF were used (Effigy four and Figure vii). The elapsing of elevated ammonium concentration for these systems was four days and eight days. The results during the experiment are divergent from the experiment carried out by Sikora et al. [eighteen], in which no increase in ammonia concentration was observed. Kuhn et al. [45] reported like results. In their research, they compared the effects of biological filters that were inoculated with nitrifying bacterial cultures with uninoculated filters. In unvaccinated RAS systems, ammonium nitrogen concentrations quickly rose above two.5 mg/dm3 and then decreased. The time in which the ammonium nitrogen concentration was increasing and decreasing differed for the studied filters. The curves of changes in concentration were also different. In the example of filters inoculated with bacterial cultures, the course was like to that observed in the organisation, which was restarted. This example supports the proposition that nitrifying bacterial cultures remain in this organisation despite disinfection with potassium permanganate. For studies based on nitrifiers immobilized in PVA (polyvinyl alcohol) and then adapted to the salty environment [51], the time needed to remove the elevated TAN concentration (initial concentration of TAN introduced into bioreactors was ten mg/dm3) to nitrite ranged from 22 days (salinity thirty ppt) to 26 days (salinity 7.5 ppt). These are longer periods than obtained during the author'southward experiment. In 2015, Hu et al. [52] conducted enquiry on the removal of nitrogen compounds from tilapia culture (Oreochromis niloticus) using aquaponic crops (tomato Lycopersicon esculentum and pak choi Brassica campestris L. subsp. chinensis). In these studies, they used root systems of cultured plants as growth surfaces for nitrifying leaner. During the study, the highest TAN concentrations were reached when the TAN concentrations peaked effectually twenty-four hours vii and were around 25 mg/dm3 (lycopersicon esculentum) and 32.5 mg/dm3 (pak choi). These concentrations are higher than those observed in the discussed experiment; moreover, they did not decrease to a similar caste in a comparable menstruum of fourth dimension. This difference is probably due to the smaller root surface area in relation to the effective surface of the applied biological filter medias.
According to Karpinski et al. [53], the increment in nitrite concentration is delayed in relation to the increase in ammonium concentration (Figure 4, Figure 5, Figure 7 and Figure viii; Tabular array i, Table 3 and Tabular array 6a). In the conducted experiment, an increase in nitrite concentrations was observed from the starting time of the experiment (Effigy v and Figure 8). Initially, these concentrations were relatively low compared to the observed concentrations of ammonium nitrogen, nonetheless, since the growth curve is exponential, the concentration of nitrite increased very chop-chop (Table 6a). Subsequently, the increase in nitrite concentration slowed down and slightly collapsed, followed by a reversal of the tendency observed in the first catamenia. Nitrite concentrations in all RAS systems dropped rapidly, reaching a relatively constant level (Figure 5 and Figure viii, Table 6a,b). This course of concentration changes was observed in all RAS systems except the organisation in which the failure occurred (Table 6a). In this organization, the menstruation of deceleration and collapse of the upward trend does not occur (Table 6b). Maximum concentration values were reached much faster and they began to decrease faster. This is reflected in the equations describing the course of nitrification (Tabular array 6a,b). The fourth dimension needed to stabilize the second phase of nitrification ranged from 15 to 33 days (Figure 5 and Figure viii). This is different from the results obtained by Kuhn et al. [45]. Despite the fact that measurements were carried out for 28 days, no downward tendency was observed. This situation occurred in systems not inoculated with nitrifying bacteria. Different results were obtained in systems inoculated with nitrifiers—no increase in nitrite concentration was observed. In the research conducted past Sikora et al. [eighteen], the time needed to stabilize the 2d stage of nitrification was 35 days, which is longer than the results observed in this commodity, although similar. Comparable times to those obtained during the experiment and to those obtained by Sikora et al. [eighteen] were too obtained by Seo et al. [51] during an experiment with the acclimatization of nitrifiers to saltwater conditions. The acclimatization time of biological filters, and thus the fourth dimension needed for nitrite oxidation to a safe level, was achieved later 33 days (salinity seven.5 and 15 ppt) and 39 days (salinity 30 ppt). Longer nitrite elimination times than observed during the experiment were also reported by Hu et al. [52]. The fourth dimension needed for oxidation of nitrite to nitrate in aquaponic cultivation was near 40 and fifty days for tomato and pak choi respectively. As in the case of ammonia oxidation, the surface of the roots on which nitrifying leaner developed was smaller than in the tested biological filter medias.
For nitrates, the recorded concentration values increased from the very commencement of the experiment. Initially, every bit with nitrites, this increase was slow to eventually accelerate. After a menses of dynamic growth, nitrate concentrations stabilized (Figure six and Figure 9; Table 7). For CF, despite the like nature of the increase curve, the concentrations were higher during the first function of the experiment. No similar trend was observed for the aforementioned type of filter in the 2nd part of the experiment (Figure six and Figure 9). Higher values in the initial phase of growth in the second part of the experiment show the concentrations in the RAS arrangement in which the failure occurred (Effigy nine). The similar nature of the increase in nitrate concentration was reported by both Kuhn et al. [45] (apportionment vaccinated with nitrifying bacteria) and Sikora et al. [18]. Kuhn et al. [45] did not observe an increment in the concentration of nitrates in the RAS system inoculated with nitrification, which was associated with the incomplete nitrification process. The nitrate concentration increase bend presented by Seo et al. [51] was comparable to the present experiment. At the end of the experiment, a decrease in nitrate concentration was observed in private RAS systems. Probably this decrease was associated with the development of heterotrophic bacteria in the volume of water.
During this experiment, it was analysed how the concentrations of nitrogen forms in water are shaped in experimental RAS systems. On this ground, the form of the nitrification procedure was determined along with the time needed for the biological filter to mature. The maturation of the biological filter is largely dependent on the temperature at which the process takes place (Tabular array viii). In systems using cool h2o, a mature filter can be obtained after a few months of its work, while this period is significantly shorter in systems developed for the needs of thermophilic species. Some other factor that may bear on the maturation of biological filters is the presence of the desired nitrifying bacteria. Biological filters inoculated with nitrifying bacteria, operating at 25 °C, bear witness desirable properties afterwards 53 days [54]. The employ of water from tilapia culture (temperature 26.iv °C) allowed a functional biological filter to be obtained afterwards 56 days [5]. These results do not reflect the information provided by Kolman [55], who states that at xviii °C, it takes twoscore to 60 days to obtain a working biological filter. In addition, the periods given differ from those obtained by Sikora et al. [xviii]. Studies accept shown that the fourth dimension needed to mature a biological filter without inoculating the civilization bike with nitrifying bacteria cultures is 35 days at 23 °C.
The conducted tests showed that at 25 °C, without introducing nitrifying bacteria cultures into the RAS arrangement, the time needed for the filter to mature is from 21 to 33 days. If nitrifying bacteria are nowadays, this period may be shorter. In one of the RAS systems used for the experiment, a failure occurred. As a result of the failure, it was necessary to restart the RAS. This RAS was dried and disinfected in accordance with the adopted procedure. In this system (drip filter, CF), a functional biological filter was obtained on mean solar day 16.
5. Conclusions
The maturation of biological filters is, despite the overall repetitive pattern, a largely variable process. The time of private stages of nitrification and their courses differ. It largely depends on the type of filter used and its filling and prevailing weather condition. The obtained results showed the effectiveness of all tested biofilter media. The shortest fourth dimension to biofilter maturation was when submerged CF was used. However, the maximum peak concentration of nitrite was also noted when it was used. For fish culture welfare, information technology is important that nitrite concentration should exist every bit low as possible. Therefore, other tested biofilter media worked better from this bespeak of view. New technologies are beingness sought, new materials are used as filter fillings and nitrification atmospheric condition are being modified. Increasingly, breeders desire to increased product faster and faster. This involves the use of increasingly efficient filters that can be run faster. Enquiry such equally that described in this article is helpful, even necessary, in learning how the nitrification process works, the conditions that shape it and what should be done to obtain fully efficient biological filters.
In addition, the article describes an unorthodox arroyo to new materials useful as cartridges for biological filters. Both PPA and PSC are materials that are associated every bit a convenient intermediate, not a useful cartridge for biological filters. Even so, these materials proved to be effective within the assumed parameters.
Writer Contributions
The following statements should exist used "Conceptualization, Grand.S., D.K.; Methodology, M.S., J.Northward.; Software, Yard.S.; Validation, One thousand.Due south., J.N.; Formal Analysis, M.Due south.; Investigation, G.Due south.; Resource, Chiliad.South., D.K., J.Due north.; Data Curation, M.S.; Writing-Original Draft Preparation, Thousand.S.; Writing-Review & Editing, M.S., J.N., D.K.; Visualization, M.Southward.; Supervision, D.K.; Project Assistants, D.Thou. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UWM Olsztyn, project No. eighteen.610.005-110 and the projection was financially supported past Minister of Science and Higher Education in the range of the programme entitled "Regional Initiative of Excellence" for the years 2019-2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Conflicts of Interest
The authors declare no disharmonize of interest.
References
- FAO. HLEF2050 Global Agriculture. 2016. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf (accessed on 10 March 2016).
- FAO. The State of World Fisheries and Aquaculture, Contributing to Food Security and Nutrition for All; Nutrient and Agronomics System of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Timmons, Northward.; Timmons, M.B.; Ebeling, J.1000. Recirculating Aquaculture Organization (RAS) Technologies. Aquac. Magazine. 2006, 32, 32–39. [Google Scholar]
- Kristensen, T.; Åtland, Å.; Rosten, T.; Urke, H.A.; Rosseland, B.O. Important influent-h2o quality parameters at freshwater production sites in two salmon producing countries. Aquac. Eng. 2009, 41, 53–59. [Google Scholar] [CrossRef]
- Greiner, A.D.; Timmons, M.B. Evaluation of the nitrification rates of microbead and trickling filters in an intensive recirculating tilapia product facility. Aquac. Eng. 1998, 18, 189–200. [Google Scholar] [CrossRef]
- Chen, S.; Ling, J.; Blancheton, J.P. Nitrification kinetics of biofilm equally affected past water quality factors. Aquac. Eng. 2006, 34, 179–197. [Google Scholar] [CrossRef]
- Emparanza, E.J.Yard. Problems affecting nitrification in commercial RAS with fixed-bed biofilters for salmonids in Chile. Aquac. Eng. 2009, 41, 91–96. [Google Scholar] [CrossRef]
- Eding, E.H.; Kamstra, A.; Verreth, J.A.J.; Huisman, E.A.; Klapwijk, A. Design and operation of nitrifying trickling filters in recirculating aquaculture: A review. Aquac. Eng. 2006, 34, 234–260. [Google Scholar] [CrossRef]
- Wicks, B.J.; Joensen, R.; Tang, Q.; Randall, D.J. Swimming and ammonia toxicity in salmonids: The effect of sub lethal ammonia exposure on the swimming operation of coho salmon and the acute toxicity of ammonia in pond and resting rainbow trout. Aquat. Toxicol. 2002, 59, 55–69. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T.K.Northward. Ammonia toxicity in fish. Marine Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Thurston, R.V.; Russo, R.C. Acute toxicity of ammonia to rainbow trout. Trans. Am. Fish. Soc. 1983, 112, 696–704. [Google Scholar] [CrossRef]
- Thurston, R.V.; Russo, R.J.; Luedtke, R.J.; Smith, C.East.; Meyn, E.L.; Chakoumalos, C.; Wang, K.C.; Dark-brown, C.J.D. Chronic toxicity of ammonia to rainbow trout. Trans. Am. Fish. Soc. 1984, 113, 56–73. [Google Scholar] [CrossRef]
- Wood, C.M. Toxic responses of the gill. In Target Organ Toxicity in Marine and Freshwater Teleosts; Schlenk, D., Benson, Due west.H., Eds.; Taylor & Francis: London, United kingdom of great britain and northern ireland, 2001; Book 1, pp. 1–89. [Google Scholar]
- Cheng, Due west.; Hsiao, I.S.; Chen, J.C. Upshot of nitrite on allowed response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Dis. Aquat. Org. 2004, lx, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Miron, D.S.; Moraes, B.; Becker, A.Thou.; Crestani, G.; Spanevello, R.; Loro, V.Fifty.; Baldisserotto, B. Ammonia and pH furnishings on some metabolic parameters and gill histology of silverish catfish, Rhamdiaquelen (Heptapteridae). Aquaculture 2008, 277, 192–196. [Google Scholar] [CrossRef]
- Benli, A.C.K.; Köksal, M.; Özkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.F.; Cancino-Madariaga, B. Ammonia retention capacity of nanofiltration and contrary osmosis membranes in a non-steady state system, to be apply in recirculation aquaculture systems (RAS). Aquac. Eng. 2014, 58, 29–34. [Google Scholar] [CrossRef]
- Sikora, Grand.; Nowosad, J.; Biegaj, Chiliad.; Kucharczyk, D.; Dębowski, M. The possibility of application of agglomerate elastomers (EPP) as media for biological bed in aquaculture. Aquac. Res. 2018, 49, 2988–2994. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, S. The impact of temperature on nitrification rate in fixed picture biofilters. Aquac. Eng. 2002, 26, 221–237. [Google Scholar] [CrossRef]
- Suhr, K.I.; Pedersen, P.B. Nitrification in moving bed and fixed bed biofilters treating effluent water from a large commercial outdoor rainbow trout RAS. Aquac. Eng. 2010, 42, 31–37. [Google Scholar] [CrossRef]
- Hamlin, H.J. Nitrate toxicity in Siberian sturgeon (Acipenser baeri). Aquaculture 2006, 253, 688–693. [Google Scholar] [CrossRef]
- Malone, R.F.; Pfeiffer, T.J. Rating fixed-film nitrifying biofilters used in recirculating aquaculture systems. Aquac. Eng. 2006, 34, 389–402. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new information for freshwater invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) assay: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Ling, J.; Chen, South. Bear on of organic carbon on nitrification operation of different biofilters. Aquac. Eng. 2005, 33, 150–162. [Google Scholar] [CrossRef]
- Guedart, T.C.; Losordo, T.G.; Classen, J.J.; Osborne, J.A.; DeLong, D.P. An evaluation of commercially available biological filters for recirculating aquaculture systems. Aquac. Eng. 2010, 42, 38–49. [Google Scholar] [CrossRef]
- Guedart, T.C.; Losordo, T.One thousand.; Classen, J.J.; Osborne, J.A.; De Long, D.P. Evaluating the effects of organic carbon on biological filtration functioning in a big scale recirculating aquaculture system. Aquac. Eng. 2011, 44, 10–18. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Targońska, K.; Hliwa, P.; Gomułka, P.; Kwiatkowski, M.; Krejszeff, S.; Perkowski, J. Reproductive parameters of common carp (Cyprinus carpio L.) spawners during natural flavour and out-of-flavor spawning. Reprod. Biol. 2008, eight, 285–289. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Nowosad, J.; Łuczyński, M.J.; Targońska, G. New technique for fertilizing eggs of burbot, asp and ide nether hatchery conditions. Anim. Reprod. Sci. 2016, 172, 143–147. [Google Scholar] [CrossRef]
- Nowosad, J.; Kucharczyk, D.; Biłas, M.; Palińska-Żarska, Grand.; Krejszeff, S. Optimization of feeding rate of juvenile common bother, (Cyprinus carpio 50.), during short intensive rearing nether controlled atmospheric condition. Experiment 2013, fifteen, 1056–1063. [Google Scholar]
- Nowosad, J.; Żarski, D.; Biłas, M.; Dryl, Chiliad.; Krejszeff, S.; Kucharczyk, D. Dynamics of ammonia excretion in juvenile common tench, Tincatinca (Fifty.), during intensive rearing under controlled conditions. Aquac. Int. 2013, 21, 629–637. [Google Scholar] [CrossRef]
- Svobodová, Z.; Máchová, J.; Poleszczuk, G.; Hòda, J.; Hamáâková, J.; Kroupová, H. Nitrite poisoning of fish in aquaculture facilities with water-recirculating systems. Acta Vet. Brno 2005, 74, 129–137. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Smith, S.A.; Boardman, Grand.D.; Angier, M.W.; Marsh, L.; Picture show, G.J., Jr. Chronic toxicity of nitrate to Pacific white shrimp. Litopenaeus vannamei: Impacts on survival, growth, antennae length, and pathology. Aquaculture 2010, 309, 109–114. [Google Scholar] [CrossRef]
- Tanikawa, D.; Nakamura, Y.; Tokuzawa, H.; Hirakata, Y.; Hatamoto, M.; Yamaguchi, T. Effluent treatment in an aquaponics-based closed aquaculture system with single-phase nitrification–denitrification using a down-menstruation hanging sponge reactor. Int. Biodeterior. Biodegrad. 2018, 132, 268–273. [Google Scholar] [CrossRef]
- Losordo, T.Thousand.; Hobbs, A.O. Using computer spreadsheets for h2o menstruation and biofilter sizing in recirculating aquaculture production systems. Aquac. Eng. 2000, 23, 95–102. [Google Scholar] [CrossRef]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for sustainable production. Aquaculture 2007, 270, 1–xiv. [Google Scholar] [CrossRef]
- Hargrove, L.50.; Westerman, P.W.; Losordo, T.M. Nitrification in 3-stage and single-stage floating bead biofilters in a laboratory-scale recirculating aquaculture system. Aquac. Eng. 1996, 15, 67–80. [Google Scholar] [CrossRef]
- van Rijn, J. The potential for integrated biological treatment systems in recirculating fish civilization. Rev. Aquac. 1996, 139, 181–201. [Google Scholar] [CrossRef]
- Ridha, G.T.; Cruz, Due east.1000. Consequence of biofilter media on water quality and biological operation of the Nile tilapia Oreochromis niloticus L. reared in a uncomplicated recirculating system. Aquac. Eng. 2001, 24, 157–166. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, S. An experimental study on nitrification biofilm performances using a series reactor system. Aquac. Eng. 1999, 20, 245–259. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Targoñska, Yard.; Chyła, B.; Dobrołowicz, A. Dynamics of changes in nitrogen and phosphorus compounds during intensive rearing of ide, leuciscusidus (L.), in a recirculating system. Curvation. Pol. Fish. 2008, 16, 459–467. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Targońska, K.; Krejszeff, S.; Czarkowski, T.; Babiarz, East.; Nowosielska, D.B. Dynamics of nitrogen and phosphorus in airtight and semi-airtight recirculating aquaculture systems during the intensive civilization of goldfish, Carassius auratus auratus (50.), juveniles. Arch. Pol. Fish. 2010, 18, 187–193. [Google Scholar] [CrossRef]
- Ebeling, J. Biofiltration. In Presentation Notebook of the Aquacultural Engineering Society Workshop; Intensive Fin-fish Systems and Technologies: Orlando, FL, USA, 2001; pp. 47–56. [Google Scholar]
- Itoi, S.; Ebihara, N.; Washio, S.; Sugita, H. Nitrite-oxidizing bacteria, nitrospira, distribution in the outer layer of the biofilm from filter materials of a recirculating water system for the goldfish Carassius auratus. Aquaculture 2007, 264, 297–308. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Drahos, D.D.; Marsh, 50.; Film, G.J., Jr. Evaluation of nitrifying bacteria product to improve nitrification efficacy in recirculating aquaculture systems. Aquac. Eng. 2010, 43, 78–82. [Google Scholar] [CrossRef]
- Pulkkinen, J.T.; Eriksson-Kallio, A.M.; Aalto, S.L.; Tiirola, M.; Koskela, J.; Kiuru, T.; Vielma, J. The effects of different combinations of stock-still and moving bed bioreactors on rainbow trout (Oncorhynchus mykiss) growth and health, water quality and nitrification in recirculating aquaculture systems. Aquac. Eng. 2019, 85, 98–105. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Williams, C.; Summerfelt, Southward.T. Evaluating the chronic furnishings of nitrate on the wellness and operation of postal service-smolt Atlantic salmon Salmo salar in freshwater recirculation aquaculture systems. Aquac. Eng. 2017, 79, i–eight. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Michaels, J.T.; Beaulaton, C.G.; Graham, W.F.; Dutt, West.; Steinbach, P.; Losordo, T.M.; Schrader, K.K.; Main, G.Fifty. Comparing denitrification rates and carbon sources in commercial scale upflow denitrification biological filters in aquaculture. Aquac. Eng. 2008, 38, 79–92. [Google Scholar] [CrossRef]
- delos Reyes, A.A., Jr.; Lawson, T.B. Combination of a bead filter and rotating biological contactor in a recirculating fish civilization organisation. Aquac. Eng. 1996, fifteen, 27–39. [Google Scholar] [CrossRef]
- Barak, Y.; van Rijn, J. Biological phosphate removal in a paradigm recirculating aquaculture treatment organisation. Aquac. Eng. 2000, 22, 121–136. [Google Scholar] [CrossRef]
- Seo, J.-Grand.; Jung, I.-H.; Kim, M.-R.; Kim, B.J.; Nam, S.-West.; Kim, S.-K. Nitrification performance of nitrifiers immobilized in PVA (polyvinyl alcohol) for a marine recirculating aquarium organisation. Aquac. Eng. 2001, 24, 181–194. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, Due south.Grand. Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol. 2015, 188, 92–98. [Google Scholar] [CrossRef]
- Karpiński, A.; Szkudlarek, Thou.; Zakęś, Z. Nitrification in recirculation systems. Practical remarks near maturation of the biologically active filter. Komunikaty Rybackie 1999, three, xi–fourteen. (In Polish) [Google Scholar]
- Sandu, S.I.; Boardman, G.D.; Watten, B.J.; Brazil, B.Fifty. Factors influencing the nitrification efficiency of fluidized bed filter with a plastic bead medium. Aquac. Eng. 2002, 26, 41–59. [Google Scholar] [CrossRef]
- Kolman, R. Effectiveness of a biological shelf filter used to treat water in a recirculation system during trout rearing. Curvation. Political leader. Fish. 1992, 1 (Suppl. one), 1–37. (In Polish) [Google Scholar]
Figure 1. Polypropylene aggregate (PPA)-specifics surface surface area—ca. 12 thou2 (A); polyethylene screw caps for PET bottles (PSC)-specifics expanse—ca. 9 mii (B); Commercial fittings HXF12KLL (CF)-specifics surface area—ca. 25.5 mtwo (C).
Figure i. Polypropylene aggregate (PPA)-specifics expanse—ca. 12 one thousand2 (A); polyethylene screw caps for PET bottles (PSC)-specifics surface area—ca. 9 mtwo (B); Commercial fittings HXF12KLL (CF)-specifics surface expanse—ca. 25.v k2 (C).
Effigy 2. Recirculation aquaculture organisation with submerged filter scheme: 1 upper retention tank—0.251 mthree, 2 rearing tank—2 × 0.096 m3, 3 lower retentivity tank (3a chamber with biofilter, 3b chamber with pump)—0.182 kiii, iv water inlets, v sprinklers, 6 water outlets, 7 pump, 8 water supply to upper retention tank, 9 submerged filter—0.030 thousand3.
Figure 2. Recirculation aquaculture arrangement with submerged filter scheme: 1 upper retention tank—0.251 thousandiii, 2 rearing tank—2 × 0.096 thousand3, iii lower retention tank (3a bedchamber with biofilter, 3b sleeping accommodation with pump)—0.182 thousandthree, 4 water inlets, 5 sprinklers, vi water outlets, vii pump, 8 water supply to upper memory tank, 9 submerged filter—0.030 miii.
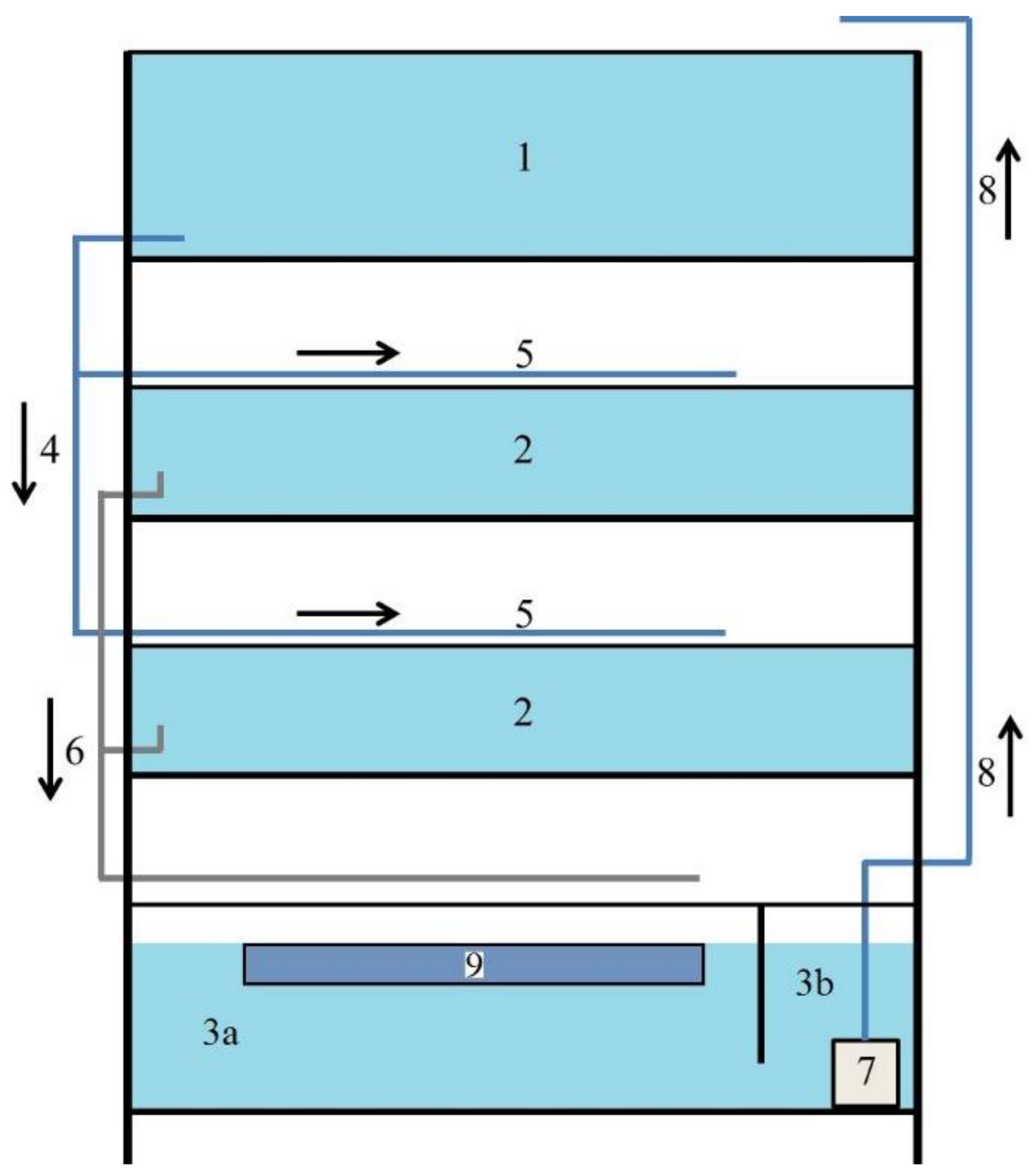
Figure 3. Recirculation aquaculture system with dripping filter scheme: 1 upper retentiveness tank—0.251 m3, ii rearing tank—2 × 0.096 one thousand3, 3 lower retention tank (3a sleeping accommodation with biofilter, 3b bedchamber with pump)—0.182 gthree, iv water inlets, v sprinklers, 6 water outlets, 7 pump, viii water supply to upper memory tank, 9 dripping filter—0.030 thousandthree.
Effigy three. Recirculation aquaculture system with dripping filter scheme: 1 upper retention tank—0.251 m3, two rearing tank—ii × 0.096 m3, three lower retentivity tank (3a chamber with biofilter, 3b chamber with pump)—0.182 g3, iv water inlets, five sprinklers, half dozen water outlets, 7 pump, viii water supply to upper retention tank, 9 dripping filter—0.030 1000iii.
Effigy 4. Ammonium nitrogen concentration in experimental systems using submerged biological filters: PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL.
Figure iv. Ammonium nitrogen concentration in experimental systems using submerged biological filters: PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL.
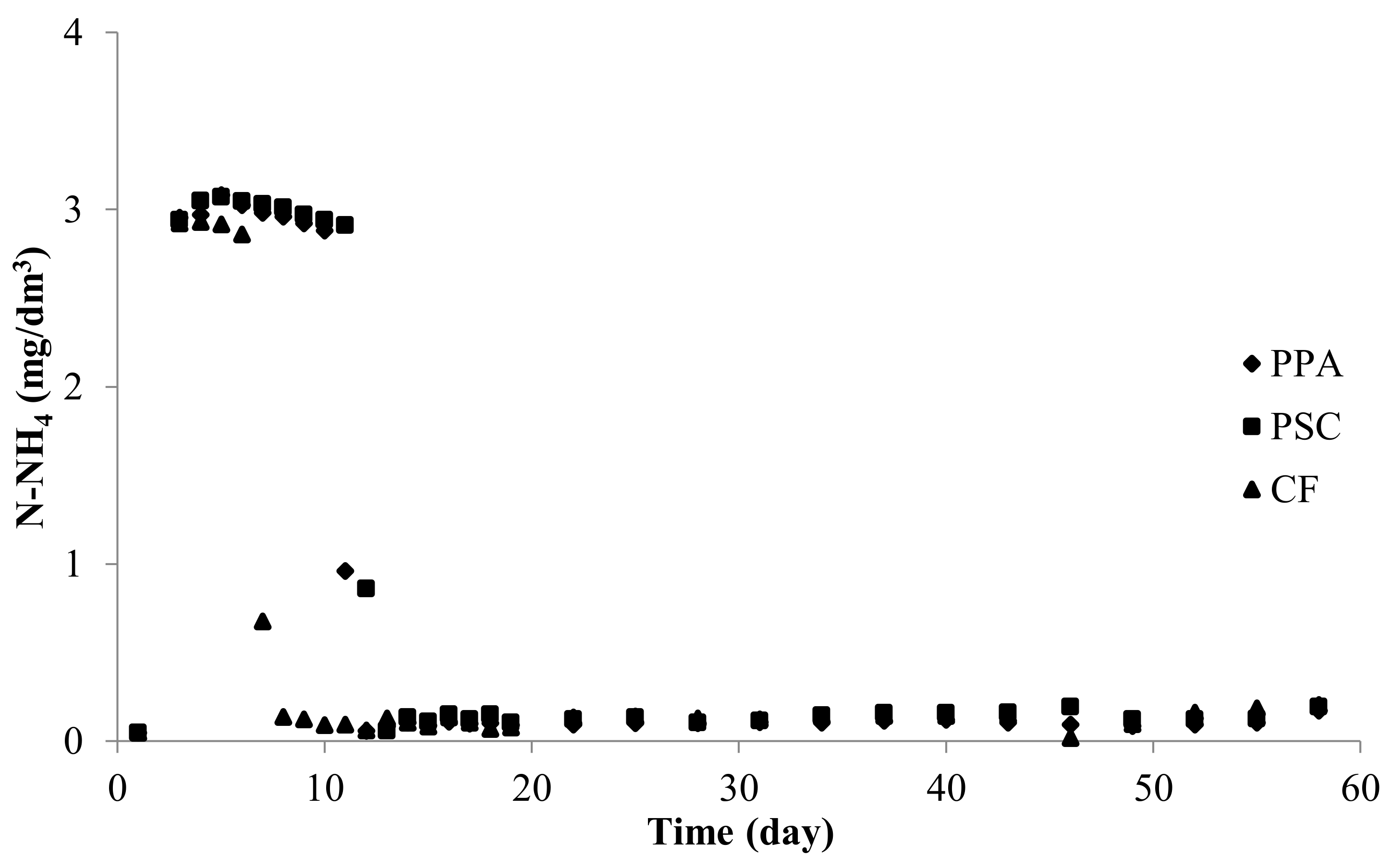
Figure five. Nitrite nitrogen concentration in experimental systems using submerged biological filters: PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL.
Figure five. Nitrite nitrogen concentration in experimental systems using submerged biological filters: PPA—polypropylene aggregate, PSC—polyethylene spiral caps for PET bottles, CF—commercial fittings HXF12KLL.
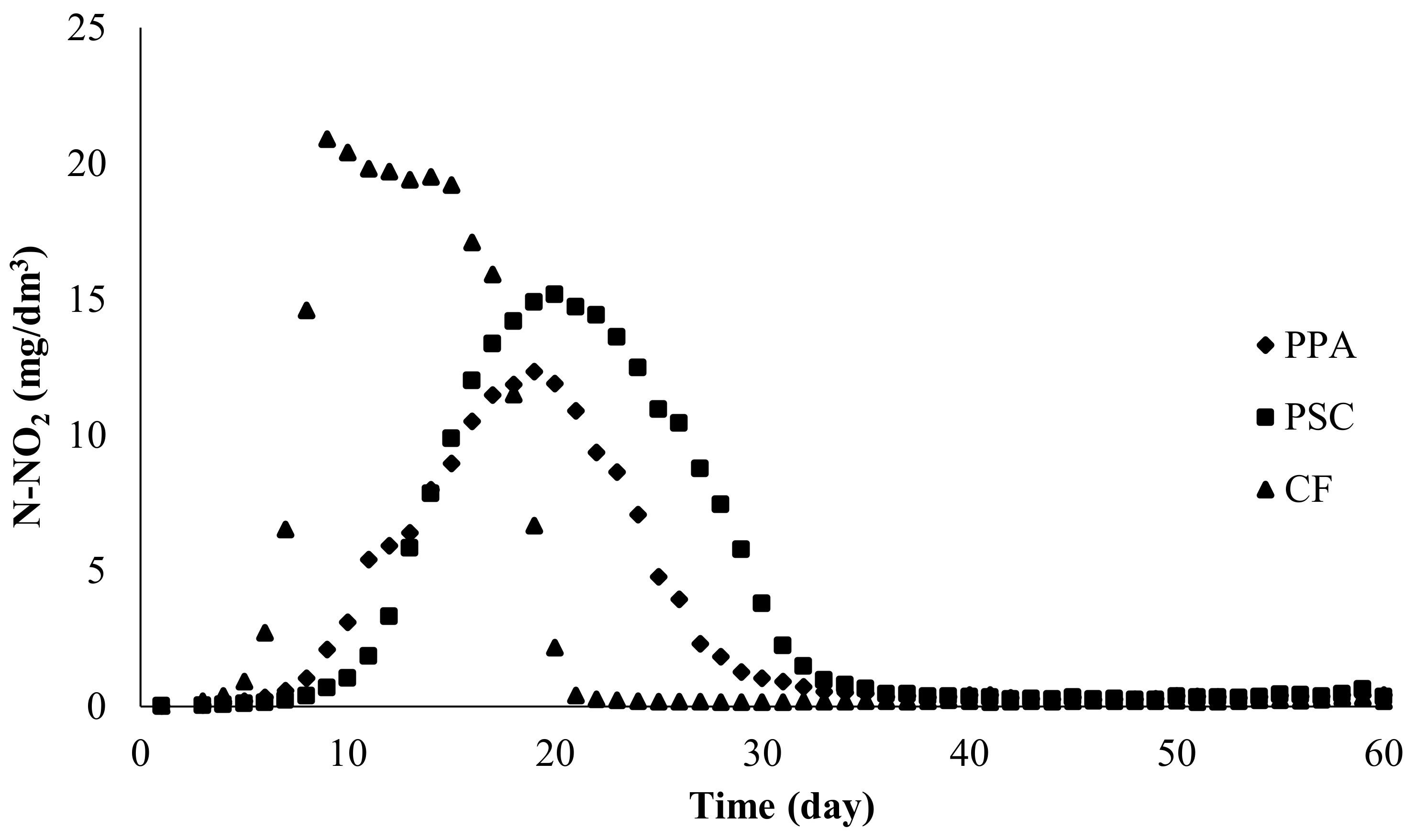
Effigy six. Nitrate nitrogen concentration in experimental systems using submerged biological filters: PPA—polypropylene amass, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL.
Effigy six. Nitrate nitrogen concentration in experimental systems using submerged biological filters: PPA—polypropylene aggregate, PSC—polyethylene spiral caps for PET bottles, CF—commercial fittings HXF12KLL.
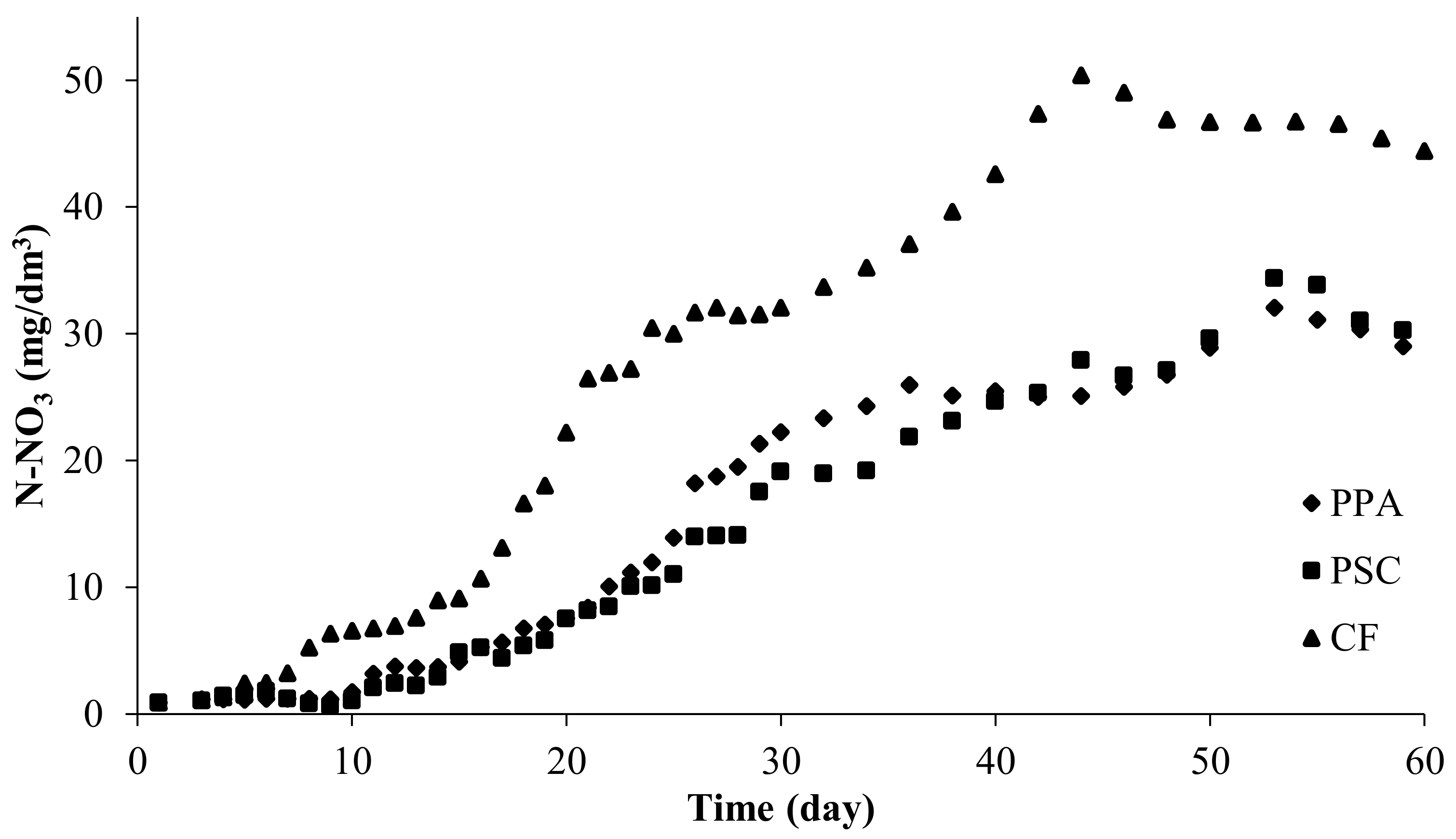
Figure seven. Ammonium nitrogen concentration in experimental systems using dripping biological filters: PPA—polypropylene amass, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—command submerged filter for dripping filters, commercial fittings HXF12KLL.
Figure 7. Ammonium nitrogen concentration in experimental systems using dripping biological filters: PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL.
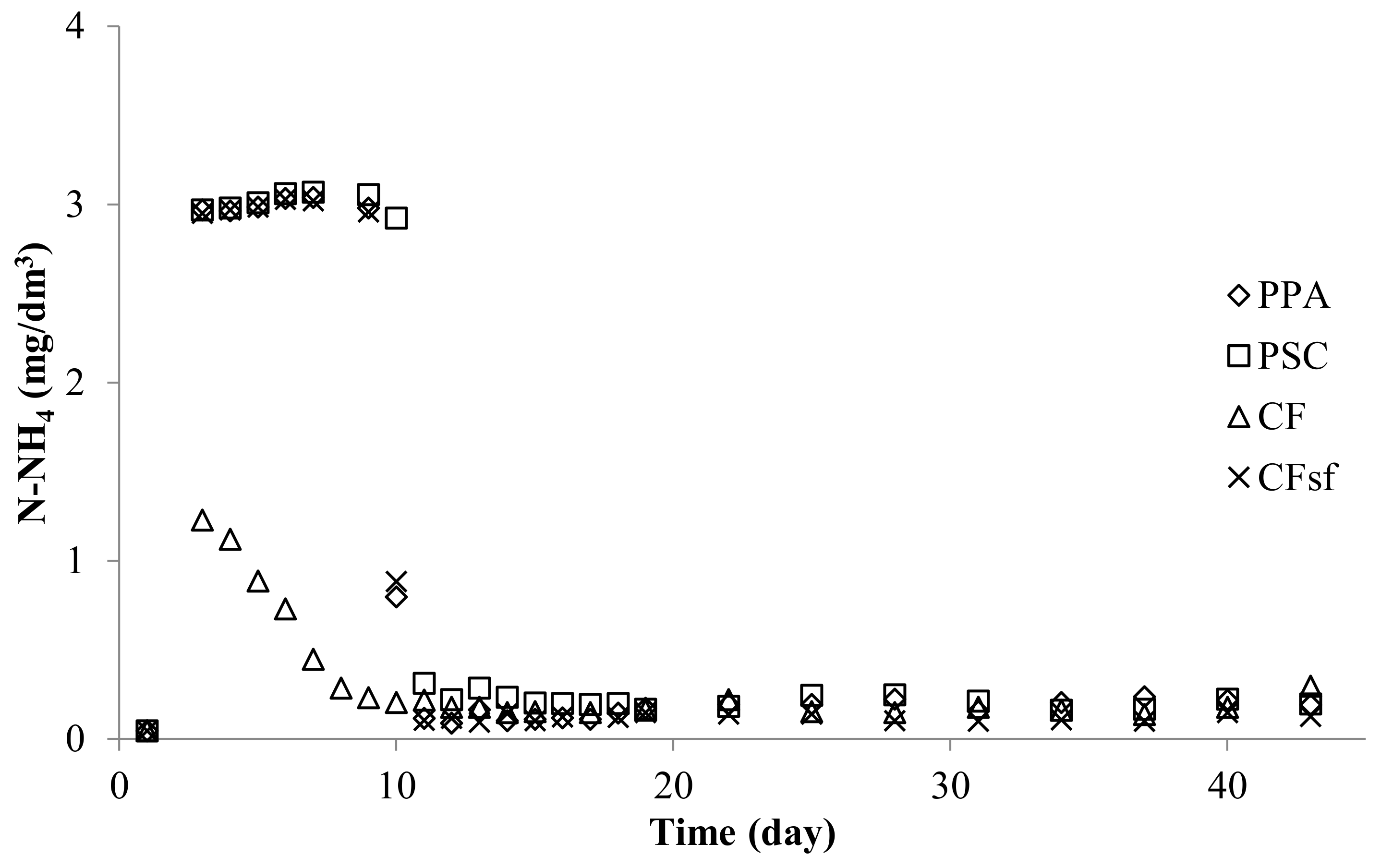
Effigy 8. Nitrite nitrogen concentration in experimental systems using dripping biological filters: PPA—polypropylene amass, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL.
Figure eight. Nitrite nitrogen concentration in experimental systems using dripping biological filters: PPA—polypropylene aggregate, PSC—polyethylene spiral caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL.
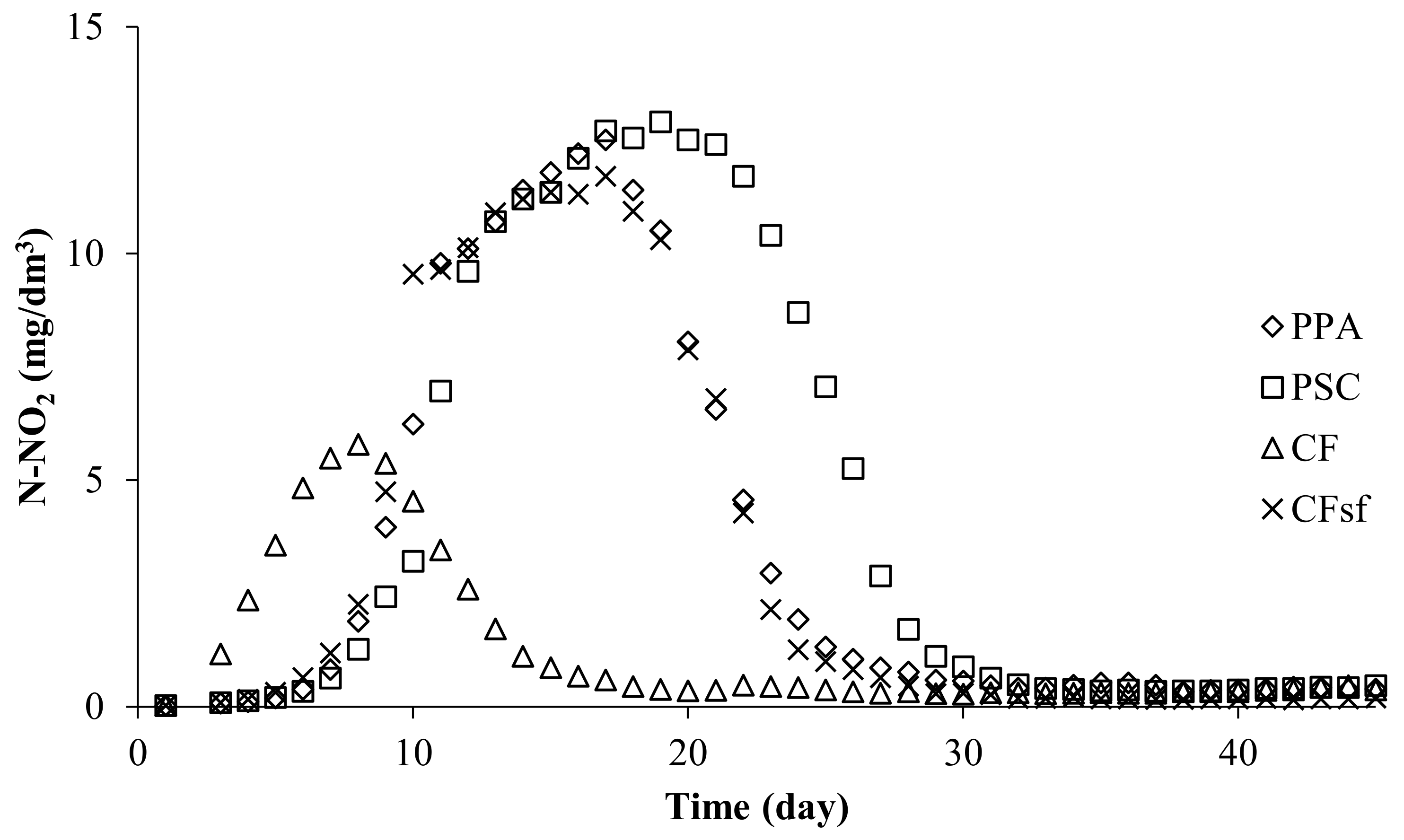
Effigy 9. Nitrate nitrogen concentration in experimental systems using dripping biological filters: PPA—polypropylene amass, PSC—polyethylene spiral caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—command submerged filter for dripping filters, commercial fittings HXF12KLL.
Figure 9. Nitrate nitrogen concentration in experimental systems using dripping biological filters: PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL.
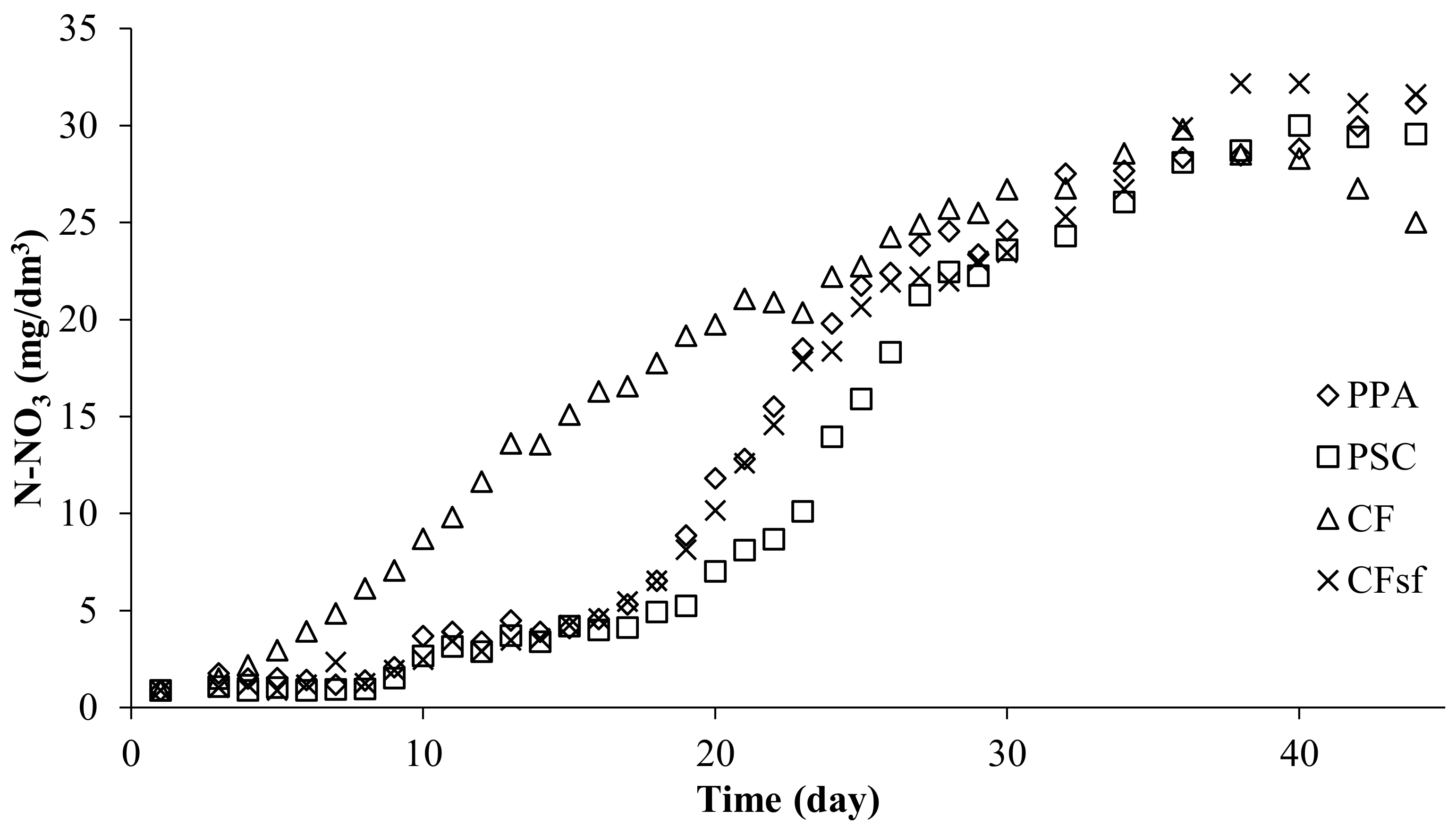
Table one. Ammonium nitrogen concentration in RAS, in which various tested fillings (PPA—polypropylene aggregate, PSC—polyethylene spiral caps for PET bottles, CF—commercial fittings HXF12KLL) were used for two separated stages, Stage I (high ammonium concentration) and Phase Two (depression concentrations ammonium) separated by a catamenia of rapid drop in ammonium (break down).
Table 1. Ammonium nitrogen concentration in RAS, in which diverse tested fillings (PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL) were used for two separated stages, Stage I (high ammonium concentration) and Stage II (depression concentrations ammonium) separated by a menstruation of rapid drib in ammonium (suspension down).
| Filling | Stage I | Break Downward | Stage 2 | |
|---|---|---|---|---|
| PPA | Average ± SD (N-NH4/dm3) | 2.971 ± 0.059 | 0.960 | 0.100 ± 0.022 |
| Duration (d) | 3–x | 11 | 12–lx | |
| PSC | Average ± SD (N-NHiv/dm3) | 2.996 ± 0.056 | 0.860 | 0.133 ± 0.031 |
| Elapsing (d) | three–11 | 12 | 13–60 | |
| CF | Average ± SD (North-NH4/dm3) | 2.906 ± 0.029 | 0,675 | 0.118 ± 0.040 |
| Duration (d) | 3–six | 7 | viii–lx |
Table two. Nitrite nitrogen concentration in RAS, in which diverse tested fillings (PPA—polypropylene amass, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL) were used for two separated stages, Stage I (high nitrite concentration) and Stage 2 (low nitrite concentration).
Table 2. Nitrite nitrogen concentration in RAS, in which various tested fillings (PPA—polypropylene amass, PSC—polyethylene spiral caps for PET bottles, CF—commercial fittings HXF12KLL) were used for two separated stages, Phase I (loftier nitrite concentration) and Phase Ii (low nitrite concentration).
| Filling | Stage I | Phase Ii | |||
|---|---|---|---|---|---|
| Increase (d) | Maximum Concentration (N-NO2/dmthree) | Decrease (d) | Average ± SD (N-NO2/dm3) | Duration (d) | |
| PPA | 1–19 | 12.340 | 19–31 | 0.404 ± 0.137 | xiv |
| PSC | i–20 | 15.180 | twenty–33 | 0.412 ± 0.169 | 12 |
| CF | 1–9 | 20.900 | nine–21 | 0.211 ± 0.049 | 24 |
Table 3. Ammonium nitrogen concentration in RAS, in which test fillings (PPA—polypropylene aggregate, PSC—polyethylene spiral caps for PET bottles, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL) were used for two separated stages: Phase I (high ammonium concentration) and Stage II (low ammonium concentrations) separated by a period of rapid drop in ammonium concentration (break downwards).
Table 3. Ammonium nitrogen concentration in RAS, in which test fillings (PPA—polypropylene aggregate, PSC—polyethylene spiral caps for PET bottles, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL) were used for 2 separated stages: Phase I (loftier ammonium concentration) and Stage Ii (depression ammonium concentrations) separated past a period of rapid drop in ammonium concentration (intermission downwards).
| Filling | Phase I | Break Down | Stage II | |
|---|---|---|---|---|
| PPA | Boilerplate ± SD (N-NHiv/dm3) | 2.996 ± 0.033 | 0.799 | 0.162 ± 0.048 |
| Duration | 3–ix | 10 | 11–45 | |
| PSC | Boilerplate ± SD (N-NH4/dmthree) | 3.010 ± 0.055 | 0.313 | 0.209 ± 0.033 |
| Duration | 3–x | 11 | 12–45 | |
| CFsf | Average ± SD (Due north-NH4/dm3) | 2.986 ± 0.033 | 0.884 | 0.120 ± 0.019 |
| Elapsing | 3–9 | 10 | eleven–45 |
Table 4. Nitrite nitrogen concentration in RAS, in which test fillings (PPA—polypropylene amass, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—command submerged filter for dripping filters, commercial fittings HXF12KLL) were used for two separated stages: Stage I ((loftier nitrite concentration) and Phase II (low nitrite concentration).
Table iv. Nitrite nitrogen concentration in RAS, in which test fillings (PPA—polypropylene aggregate, PSC—polyethylene spiral caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL) were used for two separated stages: Phase I ((high nitrite concentration) and Stage Ii (depression nitrite concentration).
| Filling | Stage I | Stage II | |||
|---|---|---|---|---|---|
| Increase (d) | Maximum Concentration (Due north-NO2/dmthree) | Decrease (d) | Boilerplate ± SD (Northward-NO2/dm3) | Elapsing (d) | |
| PPA | 1–17 | 12.500 | 17–27 | 0.480 ± 0.139 | 18 |
| PSC | 1–19 | 12.900 | 19–thirty | 0.438 ± 0.137 | 15 |
| CF | 1–viii | 5.780 | viii–15 | 0.385 ± 0.126 | 30 |
| CFsf | 1–17 | 11.700 | 17–26 | 0.261 ± 0.178 | xix |
Tabular array five. Analysis of concentrations of nitrogen compounds using Kruskal-Wallis ANOVA examination on ranks (p > 0.05) showed differences betwixt private RAS systems. Results of a multiple comparison test of mean ranks for all samples: PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL. Data marked with the aforementioned alphabetic character in rows did not differ statistically.
Tabular array 5. Analysis of concentrations of nitrogen compounds using Kruskal-Wallis ANOVA test on ranks (p > 0.05) showed differences betwixt individual RAS systems. Results of a multiple comparing test of mean ranks for all samples: PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL. Information marked with the same letter in rows did not differ statistically.
| Submerged Filter | Dripping Filter | ||||||
|---|---|---|---|---|---|---|---|
| PPA | PSC | CF | PPA | PSC | CF | CFsf | |
| Ammonium | a | b | a | bc | c | bc | ab |
| Nitrite | bcd | bcd | a | cd | d | abc | ab |
| Nitrate | bc | bc | a | b | c | ab | bc |
Table 6. (a) Nitrite nitrogen concentration analysis in experimental RAS systems using regress equation (Reg.) and correlation coefficients (R): PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL. (b) Nitrite nitrogen concentration assay in experimental RAS systems (dripping, CF) using regress equation (Reg.) and correlation coefficient (R).
Table 6. (a) Nitrite nitrogen concentration analysis in experimental RAS systems using regress equation (Reg.) and correlation coefficients (R): PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—command submerged filter for dripping filters, commercial fittings HXF12KLL. (b) Nitrite nitrogen concentration analysis in experimental RAS systems (dripping, CF) using regress equation (Reg.) and correlation coefficient (R).
| (a) | |||||
| Type of Filter, Filling | Phase | ||||
| I | II | III | IV | ||
| Submerged, PPA | Reg. | y = 0.0137e0.5439x | y = −0.1324x2 + iv.8955x − 33.778 | y = 6E + 10x−vii.218 | y = 0.0007xii − 0.0651x + one.8372 |
| (R) | 0.9988 | 0.9538 | 0.9916 | 0.6644 | |
| Elapsing (d) | 1–11 | 11–24 | 24–36 | 36–sixty | |
| Submerged, PSC | Reg. | y = 0.0117e0.4586x | y = −0.16x2 + 6.5599x − 52.287 | y = 5E + 16x−ten.96 | y = 0.0013xii - 0.1243x + iii.1995 |
| (R) | 0.9894 | 0.9909 | 0.9931 | 0.7628 | |
| Duration (d) | 1–13 | 13–27 | 27–36 | 36–60 | |
| Submerged, CF | Reg. | y = 0.0115e0.8991x | y = −0.24x2 + five.8161x − 14.642 | y = 7E + 26x−20.47 | y = 0.0002x2 − 0.0119x + 0.4015 |
| (R) | 0.9991 | 0.9031 | 0.9817 | 0.6522 | |
| Elapsing (d) | 1–8 | viii–18 | xviii–22 | 22–60 | |
| Dripping, PPA | Reg. | y = 0.0109e0.6266x | y = −0.1126x2 + 3.5649x – 16.232 | y = 6E + 09x−half-dozen.821 | y = −0.0002x2 − 0.0057 + 0.4272 |
| (R) | 0.9953 | 0.9216 | 0.9914 | 0.4423 | |
| Duration (d) | 1–xi | 11–xix | nineteen–31 | 31–45 | |
| Dripping, PSC | Reg | y = 0.0151e0.5446x | y = −0.0698x2 + ii.5974x – eleven.5 | y = 5E + 14x−nine.983 | y = 0.002x2 − 0.1489x + 3.1431 |
| (R) | 0.9979 | 0.9807 | 0.9913 | 0.9381 | |
| Duration (d) | 1–12 | 12–22 | 22–33 | 33–45 | |
| Submerged, CFsf | Reg. | y = 0.0127e0.6536x | y = −0.0698x2 + two.1765x – five.6077 | y = 2E + 12x−eight.719 | y = 0.0013xtwo − 0.1001x + 2.1323 |
| (R) | 0.9995 | 0.9390 | 0.9914 | 0.9199 | |
| Elapsing (d) | 1–ten | 10–nineteen | xix–29 | 29–45 | |
| (b) | |||||
| Type of Filter, Filling | Stage | ||||
| I | Two | Iii | |||
| Dripping, CF | Reg. | y = −0.0351x3 + 0.469x2 – 0.8346x + 0.4202 | y = 18770x−3.647 | y = 0.001xtwo – 0.067x + one.4065 | |
| (R) | 0.9998 | 0.9917 | 0.8223 | ||
| Duration (d) | 1–9 | 9–21 | 21–45 | ||
Table seven. Nitrate nitrogen concentration assay in each experimental system using backslide equation and correlation coefficients (R): PPA—polypropylene amass, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL.
Table vii. Nitrate nitrogen concentration analysis in each experimental system using regress equation and correlation coefficients (R): PPA—polypropylene aggregate, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL.
| Type of Filter, Filling | Regress Equation | Correlation Coefficients (R) |
|---|---|---|
| Submerged, PPA | y = −0.0005x3 + 0.0392x2 − 0.1142x + 0.135 | 0.9844 |
| Submerged, PSC | y = −0.0005x3 + 0.0425x2 − 0.3631x + ane.5747 | 0.9946 |
| Submerged, CF | y = −0.0006x3 + 0.0367xtwo + 0.5992x − 2.1153 | 0.9797 |
| Dripping, PPA | y = −0.0017x3 + 0.1118xtwo − 1.1623x + 3.931 | 0.9884 |
| Dripping, PSC | y = −0.0015x3 + 0.1082xtwo − 1.3589x + 4.808 | 0.9882 |
| Submerged, CFsf | y = −0.0005x3 + 0.0174xii + 0.9072x − 1.355 | 0.9968 |
| Dripping, CF | y = −0.0015x3 + 0.1052x2 − one.1256x + three.7311 | 0.9926 |
Table eight. Comparison of time needed to mature biological filter in accordance with temperature on the case of literature data and conducted experiment: PPA—polypropylene amass, PSC—polyethylene screw caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL.
Tabular array 8. Comparison of time needed to mature biological filter in accordance with temperature on the example of literature data and conducted experiment: PPA—polypropylene amass, PSC—polyethylene spiral caps for PET bottles, CF—commercial fittings HXF12KLL, CFsf—control submerged filter for dripping filters, commercial fittings HXF12KLL.
| Temperature (°C) | Fourth dimension (d) | °D | Source of Biogens | ||
|---|---|---|---|---|---|
| Sandu, et al., 2002 | 25.0 | 53 | 1325 | No data | |
| Greiner and Timmons, 1998 | 26.4 | 56 | 1478.4 | Fish | |
| Kolman, 2002 | 18.0 | 40–lx | 720–1080 | No data | |
| Sikora et al. 2018 | 23.0 | 35 | 805 | Fish | |
| This paper | Submerged, PPA | 25.0 | 35 | 875 | Fish |
| Submerged, PSC | 25.0 | 36 | 900 | Fish | |
| Submerged, CF | 25.0 | 21 | 525 | Fish | |
| Dripping PPA | 25.0 | 37 | 924 | Fish | |
| Dripping PSC | 25.0 | 32 | 800 | Fish | |
| Submerged CFsf | 25.0 | 18 | 700 | Fish | |
| Dripping CF | 25.0 | xviii | 450 | Fish | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open admission article distributed under the terms and atmospheric condition of the Artistic Eatables Attribution (CC Past) license (http://creativecommons.org/licenses/by/iv.0/).
Source: https://www.mdpi.com/2076-3417/10/2/626/htm
0 Response to "what is a disadvantage of submerged biofilters relative to most other biofilters?"
Publicar un comentario